
#159 TMA-3
2,3,4-TRIMETHOXYAMPHETAMINE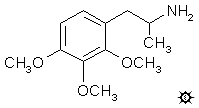
|
| [3D .mol structure] |
To a gently refluxing suspension of 3.0 g LAH in 300 mL anhydrous Et2O under a He atmosphere, there was added 3.65 g 2-nitro-1-(2,3,4-trimethoxyphenyl)propene by allowing the condensing Et2O drip into a shunted Soxhlet thimble containing the nitrostyrene and effectively adding a warm saturated solu-tion of it dropwise. Refluxing was maintained for 5 h following the completion of the addition of the nitrostyrene. The milky reaction mixture was cooled and the excess hydride destroyed by the addition of 200 mL 10% H2SO4. When the aqueous and Et2O layers were finally clear, they were separated, and 75 g of potassium sodium tartrate was dissolved in the aqueous fraction. NaOH (25%) was then added until the pH was >9, and this was then extracted with 3x75 mL CH2Cl2. Evaporation of the solvent under vacuum produced 2.5 g of a nearly colorless clear oil that was dissolved in 300 mL anhydrous Et2O which was saturated with anhydrous HCl gas. The product, 2,3,4-trimethoxyamphetamine hydrochloride (TMA-3) separated as a fine white solid. This was removed by filtration, Et2O washed, and air dried to constant weight. The yield was 1.65 g of a product which, after recrystallization from IPA, had a mp of 148-149 °C. Anal. (C12H20ClNO3) C,H.
DOSAGE: greater than 100 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 100 mg) There were no effects at all. No eye dilation, no believable diversion from complete normalcy. Appetite was normal, as well.
EXTENSIONS AND COMMENTARY: There is a small lesson to be learned from this completely inactive compound. There is no way of saying that it is or is not in-active. All that can be said is that trials were made (in this case using three separate individuals) at an oral level of 100 milligrams. And, at this level, nothing happened. And since a bottom threshold for mescaline would be perhaps 200 milligrams, it can be honestly said that the activity of this compound, if expressed relative to mescaline (using mescaline units) is less than 2 M.U. Had 200 milligrams been inactive, it would have been less than 1.0 M.U. If 2 grams had been inactive, it would have been less than 0.1 M.U. But the actual printed form, activity < 2.0 M.U. was accepted by many readers as indicating that TMA-3 was active, but at dosages greater than 100 milligrams. All that can be said is, if there is activity, then it will be at oral levels greater than 100 milligrams At the moment, as far as I know, this compound is not active in man, but then I know of no trials in excess of 100 milligrams.
This admonition applies to all the published M.U. values that are preceded by the "less than" sign, the "<."
| [ |
[Main Index] | [Forward |

