
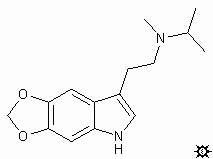
#32. 5,6-MDO-MIPT
TRYPTAMINE, N-ISOPROPYL-N-METHYL-5,6-METHYLENEDIOXY; INDOLE, 3-[2-(ISOPROPYLMETHYLAMINO)ETHYL]-5,6-METHYLENEDIOXY; N-ISOPROPYL-N-METHYL-5,6-METHYLENEDIOXYTRYPTAMINE; 3-[2-(ISOPROPYLMETHYLAMINO)ETHYL]-5,6-METHYLENEDIOXYINDOLE; 5H-1,3-DIOXOLO-[4,5-F]INDOLE-7-ETHANEAMINE, N-METHYL-N-ISOPROPYL
|
| [3D .mol structure] |
A solution of 43.8 g 2-nitro-4,5-methylenedioxybenzaldehyde in 225 mL glacial acetic acid was treated with 66.2 g nitromethane followed by 29.2 g anhydrous ammonium acetate. After being held at reflux for 2 h, the volume was reduced to approximately half by distillation, and the residues poured into ice water. The solids were removed quickly by filtration, washed well with H2O, and air-dried. An analytical sample of 2,2'-dinitro-4,5-methylenedioxystyrene was obtained as yellow crystals by crystallization from ethanol and had a mp of 121-122 °C. The unpurified isolate can be used directly in the next step.
A round-bottom flask was equipped with a mechanical stirrer, a reflux condenser, and a means of cooling with an external water bath. To 165 mL glacial acetic acid there was added 21 g 2,2'-dinitro-4,5-methylenedioxystyrene and 82 g powdered elemental iron. With good stirring, there was gentle heating applied until an exothermic reaction set in, and this was maintained at a controlled pace with external cooling. When the spontaneous reaction had subsided, the reaction was brought to reflux for 15 min, then quenched by addition to a solution of 120 g NaOH in 500 mL H2O. The reaction mixture was subjected to steam distillation and the distillate (25 L) was extracted several times with Et2O. These extracts were combined, the solvent removed under vacuum, and the residue crystallized from petroleum Et2O. There was thus obtained 4.7 g of 5,6-methylenedioxyindole as colorless plates with a melting point of 108-110 °C, for a yield of 33% of theory. Catalytic hydrogenation is an alternate process for reduction. To a solution of 19.12 g 2,2'-dinitro-4,5-methylenedioxystyrene in a mixture of 55 mL absolute ethanol, 40 mL acetic acid and 300 mL ethyl acetate there was added 4 g 10% palladium on charcoal and the reaction was shaken under 55 psi hydrogen for 45 minutes. After filtration through Celite under an inert atmosphere, the filtrate was treated with a suspension of 40 g NaHCO3 in 100 mL H2O. The organic phase was dried over anhydrous MgSO4, then the solvent was removed under vacuum. The greenish-black residue was triturated with 4x100 mL portions of boiling cyclohexane. The extracts were combined and cooled, allowing the crystallization of the product 5,6-methylenedioxyindole as a solid with a mp of 107-110 °C. The yield was 8.3 g (64%).
To a well-stirred, cold solution of 1.61 g 5,6-methylenedioxyindole in 20 mL anhydrous Et2O, there was added dropwise a solution of 1.75 mL oxalyl chloride in 5 mL Et2O. The addition took 20 min. After an additional 20 min stirring, the red crystals that formed were removed by filtration, washed with 2x5 mL Et2O, and dried under vacuum for 0.5 h. This crude acid chloride was dissolved in 100 mL anhydrous THF and cooled to 0° C with stirring under N2. An Et2O solution of N-methyl-N-isopropylamine was added until the reaction mixture remained basic (pH >9 to external pH paper). The solvents were removed under vacuum, and the residue treated with 100 mL each of H2O and CHCl3. The organic phase was separated, the aqueous phase extracted with additional CHCl3, the pooled extracts dried over anhydrous MgSO4, filtered, and the filtrate evaporated under vacuum. The residue was recrystallized from acetone to yield 1.47 g 5,6-methylenedioxy-N-methyl-N-isopropylglyoxylamide with a mp 203-204 °C. Anal: C,H,N.
To a well-stirred suspension of 1.15 g of LAH in 60 mL dry THF, there was added dropwise a solution of 1.44 g 5,6-methylenedioxy-N-methyl-N-isopropylglyoxylamide in approximately 150 mL of anhydrous THF. The mixture was brought to reflux temperature, held there for 2 h, and allowed to return to room temperature. It was hydrolyzed by the cautious addition of 1.15 mL H2O, followed with 3.5 mL 10% aqueous NaOH, and finally an additional mL of H2O. The inorganics were removed by filtration through Celite, and the filtercake was washed with additional THF. After removal of the solvent of the combined filtrate and washings under vacuum, the residue was distilled by KugelRohr and the colorless distillate recrystallized from a mixture of benzene and cyclohexane. There was thus obtained 0.43 g 5,6-methylenedioxy-N-methyl-N-isopropyltryptamine (5,6-MDO-MIPT) with a melting point of 87-89 °C (33%). Anal: C,H,N. MS (in m/z): C4C12N+ 86 (100%); indolemethylene+ 174 (7%); parent ion 260 (9%).
DOSAGE : > 50 mg, orally
DURATION : unknown
QUALITATIVE COMMENTS : (with 35 mgs, orally) "Some paresthesia noted. Nothing else."
(with 50 mgs, orally) "Maybe a trace of activity after an hour. Certainly nothing at three hours."
(with 60 mgs, orally) "There is something going on there, but I can't tell what it is. Very vague."
(with 75 mgs, orally) "Just a teasing smell of light-headedness in twenty minutes, and maybe a bit more light-headedness in an hour. I can suspect the chronology, but the character of the effects remains nebulous. It is certainly less dramatic than the 5-methoxy-compound."
EXTENSIONS AND COMMENTARY : I must continuously struggle with the reality that the substitutions on the indole ring demand an analogy to those on the phenethylamine ring. Clearly the 4-substituent is important, and the 5-substituent calls the shots (as with the 4-substituent in the phenethylamines). But is it possible that anything at the six-position is the kiss of death?
Both the 4,5-dimethoxy and the 5,6-dimethoxy-analogues are well established, and they would be fantastic tools to help unravel this problem. Clearly the 5,6-methylenedioxy materials are not too interesting, whereas the 4,5-methylenedioxy-counterparts have the rich smells of interest.
A totally compelling incident occurred in the course of writing this commentary. I decided not to assume that the reader had access to commercially available 5,6-methylenedioxyindole, but just might want to make it himself. It has been prepared from piperonal, and I said to myself, why not put into the recipe a dull but useful preparation from the ancient literature? So, let's find the 1967 article in the Monatsh. Chem. and translate the original German instructions into English for the readers. Simple and straight-forward? Yes? No!
A bit of background. Years ago, the fantastic library at the Medical School at San Francisco got into a bit of space and storage problems, and had to put its older reference issues in some sort of storage status, and it became necessary to get volumes brought out of hiding as you needed them. OK. We don't have the space. I can buy that, so let's get the space. So, a new library was designed to bring all reference material into one location and thus allow the researcher access to anything and everything he needed. Big money was asked for and big money was gotten. Finally, a single research source was created that appeared to meet all these needs. It was a multi-storeyed giant across the street from the old medical school buildings. A treasure for the medical center, with all things for all people. So I tried to find Volume 98 of Monatsh. Chem., pages including 785, published in 1967, with the details of the nitration of piperonal. No problem. The call number was W1 MO 343, so I go down three floors into the W1 territory, and I discover that there is nothing in the MO 343 section. Nothing but empty shelves. I find a helpful page who confirms that the volumes are missing, and then he asks me, "Are they pre-1975?" "Well, yes, they are." "Are they in a foreign language?" "Yes, probably in German." "Well," he says, "They have probably been moved over across the bay to Richmond, for safe keeping." "Is it a space problem?" "No, it deals with preservation and deterioration." "You are saying that older German text journals are more fragile than English counterparts?" "Well, it's a precautionary move."
The next day, I phoned in my needs to Richmond and was assured that when I got there the volume I needed would be immediately available at noon as I had asked. I made two errors in navigation in my search for the Richmond Agricultural Field Station, and located the Earthquake Research Library first. But I was met with total courtesy and was supplied with improved directions. It turned out that the field library had a Xerox machine than needed nickels, and I happened to have a pile of nickels. And I now have my recipe for the nitration of piperonal firmly in hand. But I also got a feeling that the priorities of those who needed to use reference libraries might be in conflict with the priorities of those who controlled these reference libraries.
I plead a most simple case to all of these authorities. I will in the future, on occasion, need a reference. Please let me have access to that reference when I need it. I see all these obstacles you might raise to my free access to any particular reference as a form of censorship, and I see it as a small but real measure of the superimposition of your principles upon mine. In a word, Mr. University, let me find what I want to find. Let me read what I want to read. Let me copy what I want to copy. In short, Mr. University, play the role that your founders intended you to play. My taxes paid for you; stay out of my way.
| [ |
[Main Index] | [Forward |

