
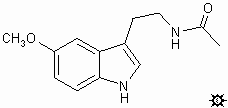
#35. MELATONIN
TRYPTAMINE, N-ACETYL-5-METHOXY; INDOLE, 3-(2-ACETAMIDOETHYL)-5-METHOXY; SEROTONIN, N-ACETYL-O-METHYL; ACETAMIDE, N-[2-(5-METHOXYINDOL-3-YL)ETHYL]; N-ACETYL-5-METHOXYTRYPTAMINE; 3-(2-ACETAMIDOETHYL)-5-METHOXYINDOLE; N-ACETYL-O-METHYLSEROTONIN; N-[2-(5-METHOXYINDOL-3-YL)ETHYL]ACETAMIDE; REGULIN
|
| [3D .mol structure] |
To a well-stirred, warm suspension of 6.0 g LAH in 100 mL anhydrous dioxane, there was added a warm solution of 3.2 g of 5-methoxy-3-indolylglyoxylamide in 100 mL of anhydrous THF. The mixture was held at reflux for 38 h, cooled, and the excess hydride decomposed by the sequential addition of wet dioxane followed by 10 mL 5% aqueous NaOH. The resulting solids were removed by filtration, and extracted several times with boiling dioxane. The filtrate and washings were combined, dried over solid KOH, stripped of solvent under vacuum yielding an oily residue. This was dissolved in 80 mL warm benzene, decolorized with charcoal, and the filtered solution treated with an anhydrous solution of HCl in EtOH until it was acidic. The precipitate that formed weighed, after air drying, 1.1 g (29%) with mp 230-235 °C. This solid was recrystallized from EtOH, which provided the product 5-methoxytryptamine hydrochloride with a melting point of 247.5-248.5 °C. Treatment with aqueous NaOH followed by the extraction and isolation of the free base, provided a fine solid that could be recrystallized from CHCl3 or EtOH, with a mp of 121-122 °C. This product has been obtained by two other procedures. The above starting indole, 5-methoxyindole, can be converted to the corresponding gramine with dimethylamine and formaldehyde, and this is converted easily with cyanide to the nitrile, 5-methoxy-3-indoleacetonitrile. This can be readily reduced to 5-methoxytryptamine with LAH. Another published procedure starts with the aldehyde of the indole, 5-methoxyindole-3-carboxaldehyde, which is coupled with nitromethane to form the nitrostyrene analogue which has been reduced in turn to the above amine with LAH. In all cases, this intermediate amine was acetylated as described below.
To a solution of 0.2 g 5-methoxytryptamine in 4 mL of glacial HOAc there was added 2.0 mL acetic anhydride and heated at steam-bath temperature for 1 min. The volatiles were removed under vacuum and the residue was ground up under a mixture of EtOH and petroleum ether to yield 0.2 g (82%) of a white solid. This, after recrystallization from an ethanol / petroleum ether mixture, provided N-acetyl-5-methoxytryptamine (melatonin) as white crystalline solid with a mp 116-118 °C. MS (in m/z): 173 (100%); indolemethylene+ 160 (97%); parent ion 232 (28%). IR (in cm-1): 713, 794, 825, 925, 1042, 1101, 1177.
DOSAGE : 1 - 10 mg, orally
DURATION : a few hours
QUALITATIVE COMMENTS : (with 2.5 mg, orally) "I took one tablet sub-lingually just before I lay down to sleep, and I slept very well. I was not tired the next day."
(with 5 mg, orally) "I cannot distinguish it from placebo."
(with 10 mg, orally) "For over a month I would take 10 milligrams every night, or five or 2.5 milligrams. More tens than 2.5's. I slept well and then I stopped it all, and still had no trouble sleeping. Why waste the money?"
EXTENSIONS AND COMMENTARY : This is a difficult drug to try to determine the active level. It is late. You want to sleep. You take a tab of melatonin and you sleep well. Or you don't take a tab of melatonin and still you sleep well. Or perhaps you sleep badly -- what connection can be drawn from the melatonin usage? The end-point of these studies is not the enhancement of consciousness but the loss of consciousness. I truly cannot say what the active level might be, because I do not know what positive experience might be expressed with an active level. In my notes is a report of a person who took 80 milligrams, orally. "Apparently I drifted quite quickly and smoothly into sleep, which was sound and which felt natural. On awakening, both my mood and performance seemed enhanced over my usual state." Is that a positive response? Melatonin has been espoused as a cure for jet-lag. But when I try to record the doses and times and effects, there is quite a bit of looseness. It is being sold in tablets (sometimes for sublingual use, why, I do not know) at dosage units from 300 micrograms to 10 milligrams. I know of one very modest i.v. trial (with 25 micrograms, at 0.10 micrograms / minute). "No subjective effects were noted."
Melatonin is found in many areas of many animals. It is involved in the skin coloration of amphibians, and in the thermal or motor regulation in some higher animals. Its major regulatory role is in response to light and, in man, is the major hormone produced by the pineal gland. This popular gland in the brain (incidentally the only unpaired site in the brain) has long been the darling of the new-age set as it is the so-called third eye. Its primary hormone, melatonin, has been the subject of many studies related to brain function. It has effects on other brain bodies that are themselves involved in hormone secretion. It has been implicated in behavioral and emotional changes in man, including anxiety, seasonal depression, and delayed sleep-phase syndrome (DSPS, with a delay in getting to sleep, and delay in coming awake). Its function is strongly affected by exposure to light, and it has been referred to as the body's hormone of darkness. And there is no question but that the biochemistry of the brain allows it to know what time of day it is. Studies with the pineal in the rat have shown that the enzymatic activity needed to run the acetylation reaction (using N-acetyltransferase, which produces melatonin from its original serotonin precursor) is 45 times more active at 10:00 PM than it is at 10:00 AM.
There has been no satisfactory pharmacology ascribed to melatonin. At low dosages it certainly decreases sleep latency. It is not a sedative at the low milligram levels (which achieves blood levels in the physiological range) but rather is a factor that might guard the user from the disruptions known as jet-lag, which is certainly a close cousin to the DSPS. Here the dosages usually explored are in the 2 to 10 milligram range. It is invariably offered as a dietary supplement rather than a sleep aid (which would be a medical claim) but a side-effect that the user is warned against is drowsiness. One popular brand I know of is available in 2.5 milligram tablets recommended for sub-lingual use. I inquired of them to learn what studies were available that indicated any virtue the sublingual route might have over direct oral use, and I learned nothing. Just a few days ago I was shown a fascinating sham offering in the OTC world. Alice brought home from the local branch of a national chain drug store a container containing 120 tablets that contained 300 micrograms each. The label said, "University Tested Strength" and "Preferred Dosage." The recommendation was for the user to take from one to three tablets (still less than a milligram). This is an example of drug-abuse at the corporate level. At relatively large dosages (75-80 milligrams) it appears to produce an increase in total sleep, along with a decrease in daytime sleepiness. This is all without hangover. It appears to be a sleep catalyst at modest levels, and a soporific at higher levels, where it can be administered chronically (75 mg/day) for a couple of weeks. with satisfactory effects. There is a paucity of reports at intermediate dosage levels. Whatever the effective dosage might be, the sales of melatonin are truly booming in the health food stores. Genzyme, a major manufacturer of melatonin, estimates that 20 million people in the United States bought melatonin for the first time in 1995, and it places the retail sales at between $200 million and $350 million per year.
In an entirely different area of pharmacology, one of the most effective protections against external radiation is a simple sulfur compound, mercaptoethylamine, commonly called MEA. This is a fascinating compound with the common name of cysteamine, and it has a wide variety of biological effects, both as a poison in that it causes ulcers, and as a treatment for poisoning in overdose cases involving acetaminophen (Tylenol). One of its most broadly studied properties is that of protecting an experimental animal against the damage of being exposed to radiation. It was unexpectedly observed that our essential and favorite neurotransmitter serotonin was every bit as effective as a radioprotective agent. In efforts to make this natural compound more accessible to the damaged animal, it was studied as the unacetylated O-methyl ether. This simple compound, 5-methoxytryptamine (5-MeO-T, or Mexamine) has been mentioned under the recipe for 5-MeO-DMT in its possible effects in potentiating CNS-active drugs. But here it deserves to be highlighted for its protection against radiation.
Two structural modification directions of 5-methoxytryptamine have been thoroughly explored. The Russians have published many years of work where they have modified that methyl group on the oxygen and have studied these changes in structure to changes in activity. In the United States, the research direction has exploited the observation that the acetamide derivative is also a good protective agent against radiation. And that amide is our title compound, melatonin. Extending the carbon length of the acetyl out to the amides in the hexanoic and octanoic area increases the prophylactic virtue, as does the making of an amide with a heptofluorobutyroyl group. The bigger the amide, the better the protection. Another study has shown melatonin to be very protective of the DNA in human white blood cells from gamma irradiation, even at very low concentrations. This may be due to the strong anti-oxidant properties of melatonin. And then, there are claims that exposing animals to low, chronic levels of melatonin can affect their life span.
Could all of these actions of melatonin be connected?. When one is flying at high elevations for long periods of time, one is exposed to quite a bit of solar radiation, and one also tends to get jet-lag. Melatonin, a natural hormone of the pineal gland, both protects against radiation and defuses jet-lag. Might there be a closer connection between the nuisance of jet-lag with the high altitude aspect of trans-Atlantic flights, rather than with the time-zone passage of trans-Atlantic or trans-Pacific flights? Personally I don't think so, as I don't get jet-lag (much) traveling in the Westerly direction, and I cross just as many time-zones and fly at similar altitudes. And I have not heard of jet-lag at all on North-to-South flights that may be just as long, but which do not cross many, if any time zones. New York to Santiago, or London to Cape Town, for example.
This is all pharmacology. These are answers to the question, what does the drug do? A second point must be loudly mentioned here, one that concerns the questions, "How does it do what it does, and where does it go to do it?" Allow me to tell a tale based on an old, made up, Sufi legend.
The master asked the student, "How do you follow a guide who cannot be seen, who walks through a dark forest in the middle of the night?"
The student answers, "It is simple. Let him carry a light."
"But then, " answers the teacher, "He is no longer the guide who cannot be seen."
"True, but at least I can now follow him, and I know where he goes."
"You must be aware you are following a different guide?"
The student thinks for a minute, and then says, "Yes, of course I know that, but what else can I do?"
This is the sad plight of the research pharmacologist, who is trying to plot the in vivo course of a biochemical that cannot be followed. It must be labeled somehow, with a radioactive element, but nature demands that it is one that is not a normal part of its makeup. So he says, I would like to follow melatonin through the darkness of the body but I cannot see it as there is no light. I will attach a brilliant radioactive label to it, something like an iodine 125, so I can follow it as it goes here and there. The iodine is the light that the melatonin molecule is carrying, and the light can indeed be followed, but it is a different molecule. It is no longer melatonin, it is now 2-iodomelatonin. It is a completely different guide.
It is a sad story to tell, but this subtle shape-shifting is all too often invisible to the researcher. We will learn what melatonin does, by studying its radio-iodinated derivative. We will determine the quality of our synthetic analogs by measuring the displacement they make of iodinated melatonin from the melatonin receptor. Iodomelatonin is not melatonin. It is a different compound. It has a different biochemistry and a different pharmacology. It is used in melatonin studies only because it can be seen. Melatonin itself is, by its nature, a dark traveler in a dark forest, and we still do not know how to study it directly.
There is a third point, an additional fillip that is associated with the popular use of melatonin. The history of transition of any interesting drug up the historic ladder, from availability to promotion, to broadcast usage, to spectacular claims, to prohibition, to illegality. This has always been seen as a pattern controlling drug use in our society. But will this apply to melatonin? We are midstride in this process, today. Its reputation as a sedative and life-extender within the health food store circuit grew quickly in the early 90's. A sleep article in the magazine Esquire (Michael Segall, October, 1994) advanced the expected warning of not knowing enough about it. "Until more is known, though, it's probably not a good idea to self-medicate your jet lag with melatonin. No one knows how much you should take nor about the potential side effects." So far, right on schedule. Although a great deal is known, and potential side effects have been examined, the restrictive warning label must be voiced. But just recently, a feature article has appeared in another magazine (Newsweek, August 7, 1995, by Geoffrey Cowley) that expands on its potential additional virtues, such as preventing pregnancy, boosting the immune system, preventing cancer, and extending the life span. Heavy duty. It will be interesting to see if this precipitates an FDA control action in light of potential medical claims, or a DEA control action in light of an abuse potential. Maybe the sales of the chemical will have to hit something in the megatonage area first. I have just ordered a few grams from the Aldrich Chemical Company and I can state that its availability remains intact for the moment. But, if it is restricted, thus withdrawn and made illegal, its popularity will grow with renewed vigor, and it will be instructive to observe in just what way the dynamics of the illicit market will evolve!
This is a present day example of a problem in the making, that law-makers and regulatory administrators have had to face again and again since that moment that the government decided that it was necessary to make a pretense of controlling the relationship between its citizens and their drugs. In the name of drug control, melatonin will eventually become illegal, and it will then pass totally out of any semblance of control. The fact that it is a natural component of the healthy human body will probably carry little weight in any attempt to thwart its becoming outlawed. Compounds such as bufotenine and DMT are normal components of our nervous system, but they are currently Schedule I drugs due to their reputed abuse potential and the absence of any accepted medical use.
A few words are needed here concerning the neurotransmitter serotonin. It is the immediate precursor to melatonin in the brain, and it is the, no, THE neurotransmitter that is the sine qua non of the brain. Everything centers on it, everything is explained by it, and all virtue and all damage that occurs there is because of it. It is not a brain chemical from outside the body. If you swallow a bunch of it, it passes on through the body without making it to the brain, because it is too polar to get through what is called the "blood-brain barrier." But maybe a enabled precursor just might make it. Recently there has been a wide promotion of 5-hydroxytryptophan that just might play this role. If it were to be actively transported into the brain, it might produce cerebral serotonin. But maybe not. I am just a bit overwhelmed by the beneficial steroids that are not steroid, and the smart drug that may or may not make you smart, of the hormone substitutes that might or might not make you a sexy octogenarian. The over-the-counter world is awash with materials that appear to be virtuous but which are carefully presented as being without any medical claims. Back to serotonin. It is an essential factor in our brain chemistry. Since it cannot be made elsewhere and be moved to where it is needed, it must be made on location. Most drugs are judged good or bad by their influence on the changes made of serotonin levels. This is the guide we follow because he is carrying the light. What is really happening in the brain is happening in darkness, because we have no way of seeing it.
It is my quiet hope that the psychedelic drugs will give us that guidance towards the understanding of the mind. They just might let us see that trail through the dark forest where most of the people who search choose to follow the lighted path.
| [ |
[Main Index] | [Forward |

