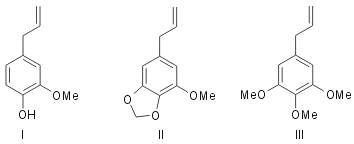
In some of the previous part1 experiments using flavones were described in support of the mechanism of phytochemical ortho-oxidation that it takes place in multiple stages. The work is now extended to simpler benzene derivatives which have biogenetic analogy. Eugenol (I) is a fundamentally important compound in nature. It has the allyl- catechol unit which may be considered to arise in the same way as the nine carbon system comprising the side phenyl nucleus and pyrone carbon atoms of flavones (see Robinson2). Myristicin (II)3 present in nutmeg, mace and parsley and elemicin (III)4, a component of elemi oil, are derivatives of allyl pyrogallol and may be considered to be related to eugenol just in the same way as myricetin is derived from quercetin and robinetin from fisetin by processes involving ortho-oxidation. These considerations have led to a simple and elegant method of synthesis of these compounds (II and III) which is the subject-matter of the present communication. The suitability of this method of ortho- oxidation for preparing methylene-dioxy compounds has already been illustrated using the sample of kanugin1.
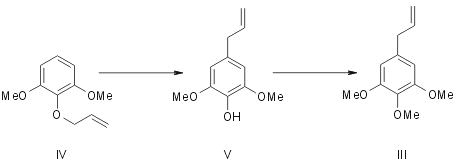
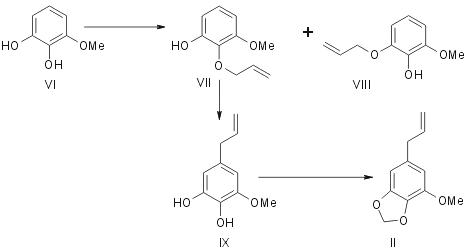
Originally Mauthner5 synthesised elemicin by subjecting 1,3-dimethyl-2-allyl pyrogallol (IV) to allyl migration and methylating the resulting hydroxy compound (V). Regarding myristicin Trikojus and White6 outlined in a preliminary note its synthesis from 1-methyl pyrogallol (VI). It gave a mixture of two liquid monoallyl ethers (VIIand VIII). They were separated and characterised as their 3,5-dinitrobenzoates. (VII) Rearranged on pyrolysis to give 3-methoxy-4,5-dihydroxy allyl benzene (IX) which was methylenated with methylene iodide to give myristicin (II).
Eugenol is now found to react with hexamine and give rise to a good yield of eugenol-5-aldehyde (X). This is a crystalline solid and exhibits the properties of an ortho- hydroxy aldehyde, forming a dinitrophenyl hydrazone and a sparingly soluble sodium salt and giving a deep blue colour with ferric chloride. In these reactions it resembles very closely orthovanillin. Its oxidation with hydrogen peroxide produces the corresponding catechol, 5-hydroxy-eugenol (IX). This is a viscous liquid, giving a green colour with ferric chloride and a yellow precipitate with lead acetate. It yields a colourless solid dibenzoate.
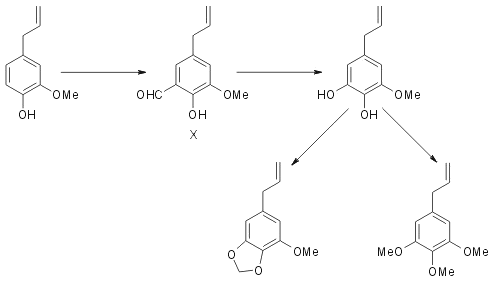
Methylenation is carried out using methylene bromide and potassium carbonate in dry acetone medium. The product is a liquid whose identity with myristicin (II) is established by a study of its properties and also by preparing the solid dibromomyristicin dibromide. Similarly methylation of the catechol (IX) using dimethyl sulphate yields elemicin. For establishing the identity of the product, besides its properties, is employed isomerisation with alkali and subsequent oxidation with permanganate yielding trimethyl gallic acid. It is interesting to note that all the stages in the above synthesis take place without noticeable isomerisation of the allyl side chain. (See Fodor7 for benzylation of eugenol without isomerisation).
Experimental
Eugenol-5-aldehyde (X)
A solution of eugenol (10 cc) in glacial acetic acid (75 mL) was treated with hexamine (40 g). The mixture was heated with shaking over a wire gauze to get a clear solution (pale brown) and was kept in a boiling water-bath for six hours. The dark brown-red solution was treated while hot with a boiling mixture of concentrated hydrochloric acid (50 cc) and water (100 cc). Heating on the water-bath was continued for another five minutes and the mixture slowly cooled. It was extracted twice with ether and the ether extract washed with water. The clear ether solution was then shaken with 20% sodium hydroxide, added cautiously in small lots of 20 to 30 cc. On shaking, the lower aqueous layer was colourless showing that only acetic acid had been extracted. Two or three such extractions removed all the acetic acid. Further addition of the alkali solution gave a bright yellow crystalline solid. After shaking vigorously the layers were allowed to separate and the lower layer diluted further with 10% alkali in order to complete the precipitation of the yellow sodium salt of eugenol-5-aldehyde. It was filtered and washed with 10% alkali and ether. It was then dissolved in excess of water and filtered and the clear filtrate acidified. After leaving overnight the pale cream coloured solid that separated out was filtered, washed well with water and dried. Yield, 3 g. The alkaline filtrate from the sodium salt was acidified and extracted with ether. The ether extract was washed with water, concentrated to a small bulk and shaken thoroughly with strong aqueous sodium bisulphite. The crystalline bisulphite addition compound was filtered and washed with ether. It was treated with dilute sulphuric acid and the mixture heated in a boiling water-bath till the solid disappeared giving oily drops. The mixture was cooled in the ice chest and the aldehyde that crystallised out was filtered. Yield, 1 g. The aldehyde samples thus obtained melted at 51-2°, when recrystallised from petroleum ether it was obtained in the form of pale yellow prisms melting at 53-54°. It was also purified by distillation under reduced pressure (20 mm), the fraction distilling at 148-50° being collected. On cooling in ice and scratching the sides of the tube, it rapidly solidified to give a mass of very pale yellow stout prisms melting at 53-4°. It was easily soluble in common organic solvents but sparingly in cold ligroin. In 10% sodium hydroxide it readily dissolved and the solution soon deposited yellow crystals of the sodium salt. With a drop of ferric chloride in alcoholic solution it gave a deep blue colour which did not change on further addition.
Dinitrophenyl-hydrazone of eugenol-5-aldehyde
An alcoholic solution of the aldehyde (0.2 g) was treated with dinitrophenyl-hydrazine (0.2 g) in alcohol (10 cc) and a few drops of concentrated hydrochloric acid. A copious crystalline precipitate of the dinitrophenyl-hydrazone was formed which was filtered after boiling for a few minutes. It was washed with alcohol and crystallised from ethyl acetate from which it separated as glistening orange red broad rectangular plates melting at 229-30°.
5-Hydroxy-eugenol (IX)
To a solution of eugenol-5-aldehyde (2.5 g) in pyridine (16 cc) was added one normal aqueous sodium hydroxide (19.5 cc) and the clear yellow solution treated dropwise with 6% hydrogen peroxide (9.6 cc) with vigorous shaking. The colour changed to red and when the solution developed turbidity a little more water was added in order to remove it. After leaving at the room temperature for 1.5 hours with occasional shaking, the solution was acidified with hydrochloric acid while cooling. The hydroxy compound separated as a heavy liquid; this separation was completed by adding common salt and the mixture was extracted thrice with ether. The ether extract was washed successively with hydrochloric acid, aqueous sodium bicarbonate and a small quantity of water. After drying over sodium sulphate it was evaporated to remove the solvent completely; a pale brown viscous oil was left behind (2.0 g). The product from three experiments was collected and distilled under reduced pressure (20 mm); the main fraction distilled at 176°. 5- hydroxy eugenol was a colourless viscous liquid. It was soluble in 5% aqueous sodium hydroxide and 10% aqueous sodium carbonate to give a deep brown solution. With a drop of ferric chloride in alcoholic solution it gave a deep violet colour changing to brownish violet and brown with another drop. After standing for a few minutes the colour changed to a stable olive green. It gave a yellow precipitate with lead acetate.
Benzoylation: dibenzoate of 5-hydroxy eugenol
The above dihydroxy compound was benzoylated by the Schotten-Baumann method. The benzoate separated from the alkaline solution in the form of a white sticky solid. It was extracted with ether, shaken well with 5% aqueous sodium hydroxide followed by water and dried over calcium chloride. On distilling off the ether a viscous liquid resulted which soon became an almost colourless crystalline solid. It was recrystallised from a mixture of ether and light petroleum from which it separated as colourless short rectangular prisms melting at 110-12°.
Methylenation: myristicin (II)
The dihydroxy compound (6 g) was dissolved in anhydrous acetone (200 cc), treated with methylene bromide (7.5 cc) and potassium carbonate (30 g) and refluxed for twenty hours. The solvent was then distilled off, the residue treated with water and the mixture extracted with ether. The ether extract was shaken with aqueous alkali, washed with water, dried over calcium chloride and distilled. The pale yellow oil left behind was distilled under reduced pressure (10 mm) when myristicin passed over at 138°. Yield, 2 g. Its refractive index was 1.5368 at 31° for white light, and 1.5343 at 30° and 1.5412 at 20° for the D line. When treated with a slight excess of bromine in petroleum ether solution (Pickles8) it yielded dibromo-myristicin-dibromide which crystallised from acetone-methyl-alcohol mixture as colourless prismatic needles and melted at 128-29° alone or in admixture with a sample made from natural myristicin.
Methylation: elemicin (III)
The dihydroxy compound (6 g) was methylated in anhydrous acetone solution (100 cc) with dimethyl sulphate (9 cc) and potassium carbonate (20 g) by refluxing for six hours. The solvent was distilled off, the residue treated with water and the mixture extracted with ether. The ether extract was shaken with aqueous alkali, washed with water, dried over calcium chloride and distilled. The residual liquid was distilled under reduced pressure (10 mm) when elemicin passed over at 148-9°. It had a refractive index of 1.5245 at 31° for white light and 1.5240 at 30° and 1.5292 at 20° for the D line. Yield, 4 g. The synthetic sample of elemicin was subjected to isomerisation with alcoholic potash and subsequent oxidation with alkaline permanganate (Semmler9). The product was found to be trimethyl gallic acid, identical with an authentic sample.
Summary
Employing the two stage process of ortho-oxidation eugenol is converted into 5-hydroxy eugenol. Methylation of this yields elemicin and methylenation myristicin. This constitutes the most convenient synthesis of these naturally occurring compounds and is highly significant form the point of view of biogenesis.
Note added in Proof: After submitting the above paper for publication, the detailed paper of Trikojus and White on the "Synthesis of Myristicin"10 was received. They have now recorded that even the isomeric pyrogallol-1-methyl-3-allyl ether (VIII) yields myristicin, apparently by migration of the allyl group to the meta-position.