
#153 3-TIM
3-THIOMESCALINE; 2,4-DIMETHOXY-3-METHYLTHIOPHENETHYLAMINE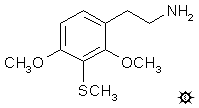
|
| [3D .mol structure] |
To a solution of 1.3 g 2,4-dimethoxy-3-(methylthio)benzaldehyde in 60 mL nitromethane there was added 0.3 g anhydrous ammonium acetate and the mixture was heated at reflux for 3 h. The hot solution was decanted from a little insoluble material, and the excess nitromethane was removed under vacuum. The residue dissolved in 10 mL hot MeOH. On cooling, yellow crystals of 2,4-dimethoxy-3-methylthio-beta-nitrostyrene were obtained which were removed by filtration and air-dried, and weighed 0.9 g. The mp was 130-133 °C and could be improved to 136-137 °C following recrystallization from MeOH (10 g/g). Anal. (C11H13NO4S) C,H.
A well-stirred solution of 0.6 g LAH in 10 mL anhydrous THF was cooled to 0 °C under He. There was added, dropwise, 0.4 mL of 100% H2SO4, followed by 0.6 g of 2,4-dimethoxy-3-methylthio-beta-nitrostyrene dissolved in a little THF. Stirring was continued for a few min as the reaction returned to room temperature, and then it was heated to a reflux for 5 min on the steam bath. The reaction was cooled again, and 25% NaOH was added dropwise until a white granular precipitate was obtained. This was removed by filtration, and the filter cake was washed with 2x25 mL Et2O. The filtrate was extracted into 25 mL dilute H2SO4 which was, in turn, made basic again and extracted with 2x25 mL CH2Cl2. The extracts were pooled, and the solvent removed under vacuum to give a residue of crude product. This was distilled from 120-140 °C at 0.3 mm/Hg yielding 0.25 g of a clear white oil. This was dissolved in 5 mL IPA, neutralized with about 3 drops of concentrated HCl, and diluted with 15 mL anhydrous Et2O. Scratching with a glass rod instigated crystallization of bright white solids which were filtered, washed with Et2O, and air dried. The weight of 2,4-dimethoxy-3-methylthiophenethylamine hydrochloride (3-TIM) was 0.2 g and the mp was 204-206 °C with decomposition. This hydrochloride appeared to be a hemihydrate. Anal. (C11H18ClNO2Sa1/2 H2O) C,H,N.
DOSAGE: greater than 240 mg.
DURATION: unknown.
QUALITATIVE COMMENTS: (with 240 mg) Briefly I thought that there might have been an alert at the 2 to 3 hour point, but I now think it was nothing. During the following day I had a mild stomach upset off and on, but I can't believe that it was connected with 3-TIM.
EXTENSIONS AND COMMENTARY: Isomescaline itself is not active, but there is no way of knowing just how "non-active" it really is. If it were to be active just beyond the levels assayed, then the introduction of a sulfur into the molecule in place of an oxygen could have increased the potency to where it might have some effect. The absence of any activity from this TIM, and the other two TIMs, might well suggest that isomescaline is really very "non-active," if that makes sense!
| [ |
[Main Index] | [Forward |

