
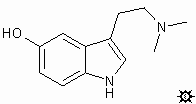
#19. 5-HO-DMT
TRYPTAMINE, 5-HYDROXY-N,N-DIMETHYL; INDOL-5-OL, 3-[2-(DIMETHYLAMINO)ETHYL]; 5-HYDROXY-N,N-DIMETHYLTRYPTAMINE; 3-(2-DIMETHYLAMINOETHYL)INDOL-5-OL; N,N-DIMETHYLSEROTONIN; BUFOTENINE; MAPPINE
|
| [3D .mol structure] |
A solution of 1.0 g 5-benzyloxyindole in 20 mL Et2O was cooled to 0 °C, vigorously stirred, and treated with 0.6 g oxalyl chloride in 10 mL Et2O, added dropwise, over the course of 0.5 h. About half way into the addition a pale red solid appeared. The stirring was continued for an additional 0.5 h and the solids were removed by filtration and washed with a small amount of Et2O. This acid chloride had a mp of 149-151 °C but was used without further purification or characterization in the following reaction. It was added in small increments to 1.2 mL of a 33% aqueous solution of dimethylamine, diluted with acidified H2O, and the resulting solids removed by filtration. These were washed with H2O, and then Et2O and air dried. The product, 5-benzyloxy-N,N-dimethyl-3-indoleglyoxylamide weighed 1.18 g (82%) when dry and had a mp of 185-187 °C.
To a well stirred suspension of 1.0 g LAH in 40 mL Et2O there was added a solution of 1.0 g 5-benzyloxy-N,N-dimethyl-3-indoleglyoxamide in 15 mL THF. When the addition was complete, the mixture was held at reflux temperature for 6 h, cooled, the excess hydride and reaction complex cautiously decomposed by the addition of H2O, and when the hydrogen evolution ceased the mixture was made basic with concentrated NH4OH. The solids were removed by filtration and the filter cake washed with THF. The filtrate and washings were combined, and the solvents removed under vacuum to give a clear residue that was dissolved in Et2O and acidified with a solution of oxalic acid in Et2O. The formed crystals were removed by filtration, washed with Et2O and air dried to yield 1.0 g (84%) of 5-benzyloxy-N,N-dimethyltryptamine oxalate with a mp of 178-180 °C after recrystallization from MeOH. The hydrochloride salt has a reported mp of 154-155 °C and of 162-163 °C.
The benzyl group was removed by hydrogenation of a solution of 0.8 g 5-benzyloxy-N,N-dimethyltryptamine oxalate in 5 mL MeOH containing 0.1 g 10% Pd/C catalyst. The mixture was shaken under three atm hydrogen for 6 h, and the solids removed by filtration. Evaporation of the solvent under vacuum gave a residue that was dissolved in anhydrous Et2O and acidified with a solution of oxalic acid in Et2O. There was thus obtained, after filtration, Et2O washing, and air drying, 0.53 g (87%) bufotenine mono-oxalate as pink needles, with a mp 93-94 °C. A mp of 178 °C in the literature may be of the bioxalate. The free base has been reported to have a mp of 125-126 °C or 146-147 °C.
DOSAGE : 8 - 16 mg, intravenously
DURATION : 1 - 2 hrs
QUALITATIVE COMMENTS : (with 1 mg, intravenously, over a three minute period) "Within a minute (from the start of the injection) I had a tight feeling in my chest and my face felt as if it had been jabbed by nettles and this lasted for about 6 minutes. I had fleeting nausea."
(with 2 mg, intravenously, over a 3 minute period) "I felt a tightness in my throat and stomach and it seemed that my pulse was racing, although apparently there was no change in either my pulse or blood pressure."
(with 4 mg, intravenously, over a 3 minute period) "During the injection, I first felt a burning sensation in my face, then a load pressing down from above, and then a numbness of the entire body. I saw red and black spots -- a vivid orange-red -- moving around. Apparently my purplish face color lasted some 15 minutes, well after my visual things had disappeared."
(with 8 mg, intravenously, over a 3 minute period) "I became lightheaded as soon as the injection started, and then my face turned purple and I became nauseated and I felt I couldn't breathe. I see white, straight lines with a black background. I can't trace a pattern. Now there are red, green and yellow dots, very bright like they were made out of fluorescent cloth, moving like blood cells through capillaries, weaving in and out of the white lines. I another two minutes, everything was pretty much gone."
(with 10 mg, intravenously, over a 50 second period) "My face was suddenly very hot. I could not breathe fast enough."
(with 10 mg, intravenously, over a 77 minute period) "There were no psychological changes."
(with 16 mg, intravenously, over a 3 minute period) "Almost immediately I felt a burning sensation in the roof of my mouth and I felt a tingling all over my body. My face turned purple, and my chest feels crushed. Everything has a yellow haze, and I was sweating heavily and I vomited. Words can't come. My mind feels crowded. When I start on a thought, another one comes along and clashes with it. I can't express myself clearly. I am here and not here. It has now been forty minutes and I feel better, but I still feel like I would like to walk it off, like a hang-over."
EXTENSIONS AND COMMENTARY : This is a presentation of the very earliest studies done with bufotenine with human subjects, studies with 14 schizophrenic patients at a state mental hospital and with two convicts in a state prison. Two convicts at a state prison were injected over the course of three minutes, with a solution of bufotenine as the salt. This single observation, a description of hyperserotoninemia (a release of serotonin in the blood, called a carcinoid flush) was all it took, at the right time and the right place, to put bufotenine on the books as a "dangerous drug" by FDA classification. And with the passage of the Controlled Substance Act of 1970, it was placed in Schedule I as a hallucinogen, with a high abuse potential and no accepted medical utility. Whatever the actual activity of bufotenine might be, and what role it could play in explaining the complex role of serotonin in the human animal, today it would be extremely difficult to study, because of the flushing of the face of an experimental subject in a prison in Maryland in study that occurred at just the wrong time.
But that is the politics of the drug. I cannot help but comment on some aspects of the medical ethics that accompanied these studies. Here were a collection of 14 schizophrenic patients, experimental cattle is the analogy that comes to mind, into which the researching physicians injected their drug. Listen to the account of one lady, following a rapid intravenous injection of bufotenine. "There was intense salivation. She could easily have drowned in her own saliva, and she had to be turned on her side. The pulse rate rose slightly during the period extending from the end of the injection until some 10 minutes later, but without much change in blood pressure. Responsiveness returned in about 23 minutes, at which time the patient was entirely lucid and, in response to a query related to a preinjection suggestion, spoke of a long repressed memory from the age of three years, when she came into the bathroom and saw her mother dying of a uterine hemorrhage. This was told without affect and had no therapeutic consequences." HOLY COW! A schizophrenic victim volunteers a long-repressed memory of her mother's traumatic death. And with the state of the healing art in the mental hospitals of that time, two physicians effectively ignore what today would be considered a dramatic break-through in therapy. Another of their trials was acknowledged as being nearly fatal, requiring artificial respiration as intervention. This is research in the healing art of medicine?
So much for the politics, and for the medical ethics lecture. What can one say about the drug itself? This is an example of a very rare breed of active compounds, one that can be found in both the animal and the vegetable kingdoms. From toads to toadstools. There are a number of extremely close structural relatives out there in the wild world. Bufotenine must first and foremost be seen as an extremely close relative to serotonin (one of our principal neurotransmitter) of which it is the N,N-dimethyl homologue). There are many modifications of it in nature (found most frequently in the skins of frogs), and these all have deceptively similar names. It is helpful to me to tally them.
Bufoviridine: This is the 1:1 ester of bufotenine with sulfuric acid. It is yet more polar than bufotenine, and correspondingly less likely to get into the brain. If the bisulfate acid position were itself esterified in some biologically stable manner, then this compound just might be centrally active, but probably only via a parenteral route as seen with 5-MeO-DMT. The exposed dimethylamino group would still make it an easy substrate for MAO's.
Bufotenidine or Cinobufagine: This is the quaternary amine internal salt, 5-hydroxy-N,N,N-trimethyltryptammonium salt. It also is frequently found as a hydrogen sulfate ester, but this latter has no trivial name. Mention has been made of bufotenidine and its sulfate ester as a occasional companion of histamine analogues found in frog skins. See the appendix on histamines.
Dehydrobufotenine: There is a covalent bond formed between the dimethylated nitrogen atom and the indolic 4-position, by the theoretical removal of a molecule of hydrogen. It is no longer a simple tryptamine but as it is a commonly found component of the chemistry of several toads, and a few giant reeds as well, it is included here. It is, by definition, a quaternary amine salt. The original structure assigned it was that of a vinylamine (with the loss of a hydrogen molecule from the alpha/beta chain positions. This was shown to be incorrect.
Bufothionine: This is the hydrogen sulfate ester of dehydrobufotenine.
O-Methylnordehydrobufotenine: This is a rearrangement product of dehydrobuftenine, which may be a natural product or it may be an artifact of analysis.
O-Methylbufotenine: This represents a true crossover alkaloid, found in many plants as well as in the toad family, It is entered as a recipe under the synonym, 5-MeO-DMT.
Norbufotenine (5-hydroxy-N-methyltryptamine, N-methylserotonin, 5-OH-NMT): This base is scattered in both the animal and the plant kingdoms. It has been found in quite a few toads and in barley shoots. It has been isolated from the herb Desmodium pulchellum. This is an interesting twilight compound lying half way between a notorious toxin (bufotenine) and a vital neurotransmitter (serotonin). And it is unexplored, for shame. It has been detected in the urine of schizophrenic subjects, but that doesn't say anything about its potential activity. That bare hydroxyl group may make it difficult to get into the brain. Probably as difficult as bufotenine itself proved to be. The removal of the second methyl group reveals serotonin.
Bufogenins or Bufagins: These are nitrogen-free steroidal lactones that are heart toxins found in toad venom. They have no chemical resemblance to bufotenine whatsoever. Examples are bufogenin B, bufotalin and bufotalinin.
Bufotoxins: These are steroidal bufagins, usually linked via an hydroxyl suberic acid which is, in turn, bound by a peptide link to arginine.
The are two structural variations of bufotenine that I feel would be interesting to explore. One deals with the ethers of the 5-hydroxyl group. The O-methyl ether is, of course, 5-MeO-DMT. It is mentioned above under the name O-methylbufotenine. What about the very obvious O-ethylbufotenine, 5-EtO-DMT? It had once been synthesized from 5-ethoxytryptophol in a physostigmine study, and had been converted to bufotenine with aluminum chloride. If the analogy from the phenethylamines applies here (MEM is as potent as TMA-2) then 5-EtO-DMT should be as potent as 5-MeO-DMT. And probably would have to be smoked for the very same reasons. Another variation deals with possible esters on that 5-hydroxyl group. Finding activity in things like the bisulfate bufoviridine would be unlikely, but perhaps an acetate ester (easily made from bufotenine and acetic anhydride) would allow it to make it into the CNS, in a manner similar to the acetate of the 4-hydroxy analogue, psilocin.
There once was (and maybe still is) a group called The Institute of Current World Affairs who gave grants to people to allow them to travel and write on topics of cultural interest. I was on their mailing list, which gave me a fabulous collection of essays and vignettes written by Andy Weil, who later spun some of them together into a book called The Marriage of the Sun and Moon. In trying to organize and understand the pharmacology of bufotenine I was pleasantly reminded of the essays Andy devoted to the magic of Uri Geller.
He was initially completely entranced by the way this young man from Israel could muster the psychic energy of an audience to bring about some remarkable phenomenon. It was not just the bending of keys and spoons, but it was remote viewing and mind-reading as well. It was the stuff of the miraculous.
Andy was a total convert, but then there was an abrupt erosion of certainty that began with Andy's meeting with a skeptic called the Amazing Randi, who could duplicate most of the illusions with his sleight of hand mastery. Andy went from total belief to total disbelief in a very short period of time. It seemed that his earlier conviction was wrong and that all was indeed misrepresentation. This change in position of course managed to offend both camps. Then he came finally to a middle ground. The status of Uri Geller may be essentially unanswerable. Psychic phenomena are believed if that is needed. Are these things factual? Who is judging it all, and from what point of view?
And so it is with bufotenine. Is it an active psychedelic? Absolutely yes, absolutely no, and maybe yes and maybe no.
The early reports used the "psychotomimetic" term and pushed for a psychedelic interpretation of the observations. Observers saw colored spots, straight lines against a black background. Words can't come. My mind feels crowded. These and similar descriptions are often encountered as components of psychedelic experiences. And yet a skeptic would point to the terms that are closely associated with toxic effects, and peripheral poisoning: my face turned purple and I became nauseated, I could not breathe fast enough. Lactimation and tachycardia. These all are exerpts from the small selection of comments given above. In the period that has followed the earliest studies described in the "Qualitative Comments" section above, there have been about a dozen additional reports that could be offered that describe the same scatter of ups and downs, employing different modes of delivery. With insuflation, I have one that claims a feeling of fear, a flushing of the face, lacrimation and tachycardia, with ten milligrams. Another report states that after snorting forty milligrams, observed neither objective nor subjective effects. Some clinicians demand that the compound is unquestionably a psychotomimetic and it must be catalogued right up there along with LSD and psilocybin. Others, equally sincere, present human trials that suggest only peripheral toxicity and conclude that there is no central action to be seen. And there are many who state that there are no effects for it at all, either inside or outside the CNS. The psychopharmacological status of bufotenine, like that of Uri Geller, may be essentially unanswerable.
Two recent publications provide new and provocative input to this dialogue. One of these involved a series of appearances of a reddish substance on the East Coast called Chinese Love Stone, Black Stone, Rock Hard or Stud 100, being sold as aphrodisiacs. They were to be moistened and rubbed on the genitals, but as might be expected, quite a few were eaten and eventually smoked. They contained steroidal toxins, and were possibly related to some frog origins, but they were claimed to be bufotenine and indeed contained bufotenine in addition to several cardiotoxins as well as 5-MeO-DMT.
A second report carries, at least for me, much more impact. A study of the use of the seeds of a South American legume, Anadenanthera colubrina var. Cebil by the Argentine Shamans in Chaco Central, shows then to be dramatically psychedelic. And yet, extremely sophisticated spectroscopic analysis has shown them to contain bufotenine and only bufotenine as their alkaloid component.
At the bottom line, I do not really know of bufotenine is a psychedelic drug. Maybe yes and maybe no.
| [ |
[Main Index] | [Forward |

