
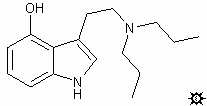
#20. 4-HO-DPT
TRYPTAMINE, 4-HYDROXY-N,N-DIPROPYL; 4-INDOLOL, 3-[2-(DIPROPYLAMINO)ETHYL]; 4-HYDROXY-N,N-DIPROPYLAMINOTRYPTAMINE; 3-[2-(DIPROPYLAMINO)ETHYL]-4-INDOLOL
|
| [3D .mol structure] |
To a stirred suspension of 0.50 g LAH in 10 mL anhydrous THF, stirred, under nitrogen and at room temperature, there was added a solution of 0.66 g 4-acetoxyindol-3-yl-N,N-dipropylglyoxylamide in 10 mL anhydrous THF. This was added dropwise at a rate that maintained the reaction at reflux. When the addition was complete, the reflux was maintained for an additional 15 min and then the reaction was cooled to 40 °C. The excess hydride and the product complex was destroyed by the addition of 1 mL EtOAC followed by 3 mL H2O. The solids were removed by filtration, the filter cake washed with THF, the filtrate and washings pooled, and the solvents removed under vacuum. The residue was distilled at the KugelRohr and the distillate recrystallized from EtOAC/hexane. Thus there was obtained 0.27 g (51%) of 4-hydroxy-N,N-dipropyltryptamine (4-HO-DPT) with a mp 96-97 °C. Anal: C,H,N.
DOSAGE : unknown
DURATION : unknown
QUALITATIVE COMMENTS : (with 20 mg, orally) "Possible threshold, nothing more."
EXTENSIONS AND COMMENTARY : Here is another case where there just aren't enough observations to determine at what level the activity will be seen, or what form it will take. The track record is pretty well established with the oxygen-free analogue DPT, and it would be hard to imagine a loss of potency by incorporating the "psilocin signature", the 4-hydroxy group. This threshold suggests something is nearby. It is a shame that the compound is so difficult to make.
| [ |
[Main Index] | [Forward |

