
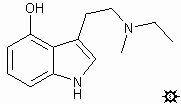
#21. 4-HO-MET
TRYPTAMINE, N-ETHYL-4-HYDROXY-N-METHYL; 4-INDOLOL, 3-[2-(ETHYLMETHYLAMINO)ETHYL]; N-ETHYL-4-HYDROXY-N-METHYLTRYPTAMINE; 3-[2-(ETHYLMETHYLAMINO)ETHYL]-4-INDOLOL
|
| [3D .mol structure] |
To 10 mL of a stirred solution of LAH (1 M in THF under N2), there was added dropwise a solution of 0.57 4-acetoxyindol-3-yl-N-ethyl-N-methylglyoxylamide in 10 mL anhydrous THF. When the addition was complete, the reaction mixture was brought to a reflux for 15 min. After cooling to 40 °C, sufficient water was added to decompose both the reaction complex and the excess hydride. After filtration through Celite (under an N2 atmosphere), the solvent was removed under vacuum, and the solid residue recrystallized from EtOAc/hexane to provide 0.18 g (41%) N-ethyl-4-hydroxy-N-methylindole (4-HO-MET) with a mp 118-119 °C. Anal: C,H,N.
DOSAGE : 10 - 20 mg, orally
DURATION : 4 - 6 hrs
QUALITATIVE COMMENTS : (with 20 mg, orally) "Qualitatively a lot like psilocin. I started within the first half-hour, and at the max, I felt the same alteration of color and form, and times, sound was felt. As with psilocin, the experience was wave-like, with an alteration of effects between near-normal perception at one minute, only to be swept up in a swirl of altered concept the next minute.
EXTENSIONS AND COMMENTARY : First, an apology for just a single entry in the comments section. This, and several other of these substituted hydroxy and methoxy tryptamines, had had earlier evaluations, but the notes are not at hand and cannot be used. Much will have to come back from memory, and there must be an appropriate fuzziness allowed for the concluded generalization as to dose and duration. With this particular compound, some of the original observations suggested that it was more potent than psilocin, certainly more dramatic. But at the bottom line, I doubt that this ethyl homologue, or the isopropyl homologue 4-HO-DIPT for that matter, could be distinguished from the methyl counterpart psilocin in any blind clinical study.
What's to choose between them? From the view-point of synthesis, the cost and availability of the secondary amine will certainly be a factor. Both methylethyl amine and methylisopropylamine are available, but are quite expensive. Dimethylamine, on the other hand, is dirt cheap but it is a recognized precursor to DMT and thus is difficult to find. In any events, the dimethyl compound is widely available in the mycological arena, and I suspect it would be simplest to stay with nature.
| [ |
[Main Index] | [Forward |

