
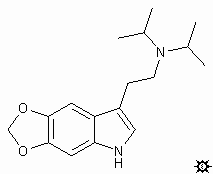
#29. 5,6-MDO-DIPT
TRYPTAMINE, N,N-DIISOPROPYL-5,6-METHYLENEDIOXY; INDOLE, 3-[2-(DIISOPROPYLAMINO)ETHYL]-5,6-METHYLENEDIOXY; N,N-DIISOPROPYL-5,6-METHYLENEDIOXYTRYPTAMINE; 3-[2-(DIISOPROPYLAMINO)ETHYL]-5,6-METHYLENEDIOXYINDOLE; 5H-1,3-DIOXOLO-[4,5-F]INDOLE-7-ETHANEAMINE, N,N-DIISOPROPYL
|
| [3D .mol structure] |
To a well stirred suspension of 0.77 g of LAH in 40 mL dry THF, there was added dropwise a solution of 0.95 g 5,6-methylenedioxy-N,N-diisopropylglyoxylamide in approximately 100 mL of anhydrous THF. The mixture was brought to reflux temperature and held there for 2 h, and allowed to return to room temperature. It was hydrolyzed by the cautious addition of 0.8 mL H2O, followed with 2.4 mL 10% aqueous NaOH, and finally an additional 0.8 mL of H2O. The inorganics were removed by filtration through Celite, and the filtercake was washed with additional THF. After removal of the solvent of the combined filtrate and washings under vacuum, the residue was distilled by KugelRohr and the colorless distillate recrystallized from a mixture of Et2O/hexane. There was thus obtained 0.52 g 5,6-methylenedioxy-N,N-diisopropyltryptamine (5,6-MDO-DIPT) with a melting point of 93-94 °C (60%). Anal: C,H,N.
DOSAGE : unknown
DURATION : unknown
EXTENSIONS AND COMMENTARY : So why enter an entry into a listing of active compound when it is simply not known if it is active or not? The truth is, that none of these three 5,6-methylenedioxy N,N-disubstituted tryptamines (this one, or 5,6-MDO-DMT or 5,6-MDO-MIPT) has been explored up to an active level, but they are appealing targets in that they have the progression of nitrogen substituents that has proven so valuable in similar sequences of compounds. This is the pattern dimethyl, methylisopropyl and diisopropyl. With both the unsubstituted, and the 5-methoxy-substituted groups, the activity goes from quite potent but requiring parenteral administration, to highly potent and orally active, and back to quite potent and orally active, as the methyl groups are progressively replaced with isopropyl groups. It would be instructive to see if this arrangement was maintained with this methylenedioxy trilogy.
The three closed ring group of substituents mentioned in the pyr-T recipe have also been described with this 5,6-methylenedioxy ring substitution. These could be named 5,6-MDO-pyr-T (the pyrrolidine analogue, mp 110-112 °C), 5,6-MDO-pip-T (the piperidine analogue, mp 150-152 °C) and 5,6-MDO-mor-T (the morpholine analogue, mp 117-119 °C). To my knowledge, none of these has ever been put into man.
| [ |
[Main Index] | [Forward |

