
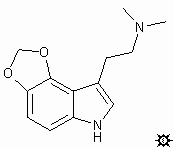
#30. 4,5-MDO-DMT
TRYPTAMINE, N,N-DIMETHYL-4,5-METHYLENEDIOXY; INDOLE, 3-[2-(DIMETHYLAMINO)ETHYL]-4,5-METHYLENEDIOXY; N,N-DIMETHYL-4,5-METHYLENEDIOXYTRYPTAMINE; 3-[2-(DIMETHYLAMINO)ETHYL]-4,5-METHYLENEDIOXYINDOLE; 5H-1,3-DIOXOLO-[4,5-E]INDOLE-7-ETHANEAMINE, N,N-DIMETHYL
|
| [3D .mol structure] |
A mixture of 21 g 2,3-methylenedioxytoluene and 1 g Hg(OAc)2 in 59 mL acetic acid was stirred and heated to 80 °C. To this there was added, dropwise, 12.8 g of concentrated nitric acid. Heating and stirring was continued for 2 h. The reaction mixture was quenched by pouring it into ice-H2O, and extracted with Et2O. The extracts were pooled, dried with anhydrous MgSO4, and the volatiles removed under vacuum. The orange solid residue was recrystallized from EtOH to provide 20 g (58%) of a mixture of the ortho- and meta-products, 2,3-methylenedioxy-5-nitrotoluene and 2,3-methylenedioxy-6-nitrotoluene, with a mp 65-67 °C. This unresolved mixture was employed in the next step without further purification.
In a flask equipped with a total reflux packed column and a variable take-off head, 15.0 g of the 2,3-methylenedioxy-5-nitrotoluene / 2,3-methylenedioxy-6-nitrotoluene mix was added to a mixture of 100 mL freshly distilled DMF and 12.8 g of N,N-dimethylformamide dimethyl acetal. The reaction mixture was heated to a controlled reflux that kept the head temperature at 50 to 70 °C allowing the removal of methanol. After 4 h, 90% of the theoretical amount of MeOH (7.4 mL) had been distilled off, and the residual solvent (DMF) was removed under vacuum. The dark residue was dissolved in benzene, washed with H2O, dried with anhydrous MgSO4, and the solvent removed under vacuum. The crude crystalline product was recrystallized from hexane / benzene to provide 4.8 g (50%) of 2,3-methylenedioxy-6-nitro-beta-dimethylaminostyrene as red needles with a mp 126-120 °C.
A solution of 3.5 g 2,3-methylenedioxy-6-nitro-beta-dimethylaminostryrene in 200 mL benzene was placed in a Parr hydrogenation bomb and treated with 0.35 g of 10% Pd / C. The mixture was shaken for 7 h under 3 atm H2. The catalyst was removed by filtration, and the filtrate was washed first with 2N H2SO4, followed by aqueous NaHCO3 and H2O. This was dried and the solvent removed under vacuum to give 1.2 g (50%) of 4,5-methylenedioxyindole as a residue. After crystallization from benzene / petroleum ether, it had a mp 111 °C. Anal: C,H,N.
A solution of 4.8 g 4,5-methylenedioxyindole in 60 mL anhydrous Et2O was stirred and cooled with an external ice bath. There was added, dropwise, a solution of 5.0 g oxalyl chloride in Et2O so that the temperature did not exceed 5 °C. The intermediate acid chloride separated as a red solid, and was removed by filtration and washed with Et2O. It was then suspended in 60 mL cold anhydrous Et2O, treated with 7 mL dimethylamine and the mixture stirred for 30 min. The solvent was decanted from the crude solids that formed, and they were suspended in 50 mL H2O. The product was removed by filtration and vacuum dried to provide 5.7 g (77%) 4,5-methylenedioxy-N,N-dimethylindole-3-glyoxylamide as a white solid with a mp 240-243 °C.
To a stirred and cooled solution of 3.8 g LAH in 100 mL anhydrous THF there was added, over the course of 1 h, a solution of 3.7 g 4,5-methylenedioxy-N,N-dimethylindole-3-glyoxylamide in 500 mL anhydrous THF. After 1 h reflux, the cooled reaction mixture was treated with 3.8 mL H2O, followed by 3.8 mL aqueous 5% NaOH and then by an additional 10.4 mL H2O. The solids were removed by filtration and washed with THF. The combined filtrate and washings were dried (MgSO4) and the solvent removed under vacuum. The residual oil was distilled at the KugelRohr (0.5 mm / Hg at 100 °C) to give a distillate that solidified. This was crystallized from benzene / petroleum ether to provide 0.25 g (8%) of 4,5-methylenedioxy-N,N-dimethyltryptamine (4,5-MDO-DMT) with mp 93-95 °C. Anal: C, H. N.
DOSAGE : unknown
DURATION : unknown
EXTENSIONS AND COMMENTARY : The two aromatic ring positions that are associated with human psychedelic activity are the 4-position (of psilocybin fame) and the 5-position (of 5-methoxy-this-and-that fame). Here is a compound with both positions oxygen-substituted (with the methylenedioxy ring that is so effective in the phenethylamine world) and it has not been looked at in man, to my knowledge. I snooped around in the literature associated with this kind of DMT substitution, and the world of di-oxygen substitution to be found at these two potent focal points is almost unknown. Aside from the 4,5-methylenedioxy-diisopropyltryptamine, described here in the recipes (under 4,5-MDO-DIPT), there are only five of these dioxygenated compounds known. 4-benzyloxy-5-methoxytryptamine is the precursor of the DMT and DET homologues with a 4-hydroxy group exposed after hydrogenation. But nowhere is there a 4,5-dimethoxy pattern. In fact the methoxy group is unknown in the 4-position in this simple system.
I have been told that Mark Julia, in France, had made the 4-hydroxy-5-methoxy compound with a methyl and an ethyl on the tryptamine nitrogen. If so, it certainly is not in the literature abstracts and thus is of unknown properties. I will search this out.
| [ |
[Main Index] | [Forward |

